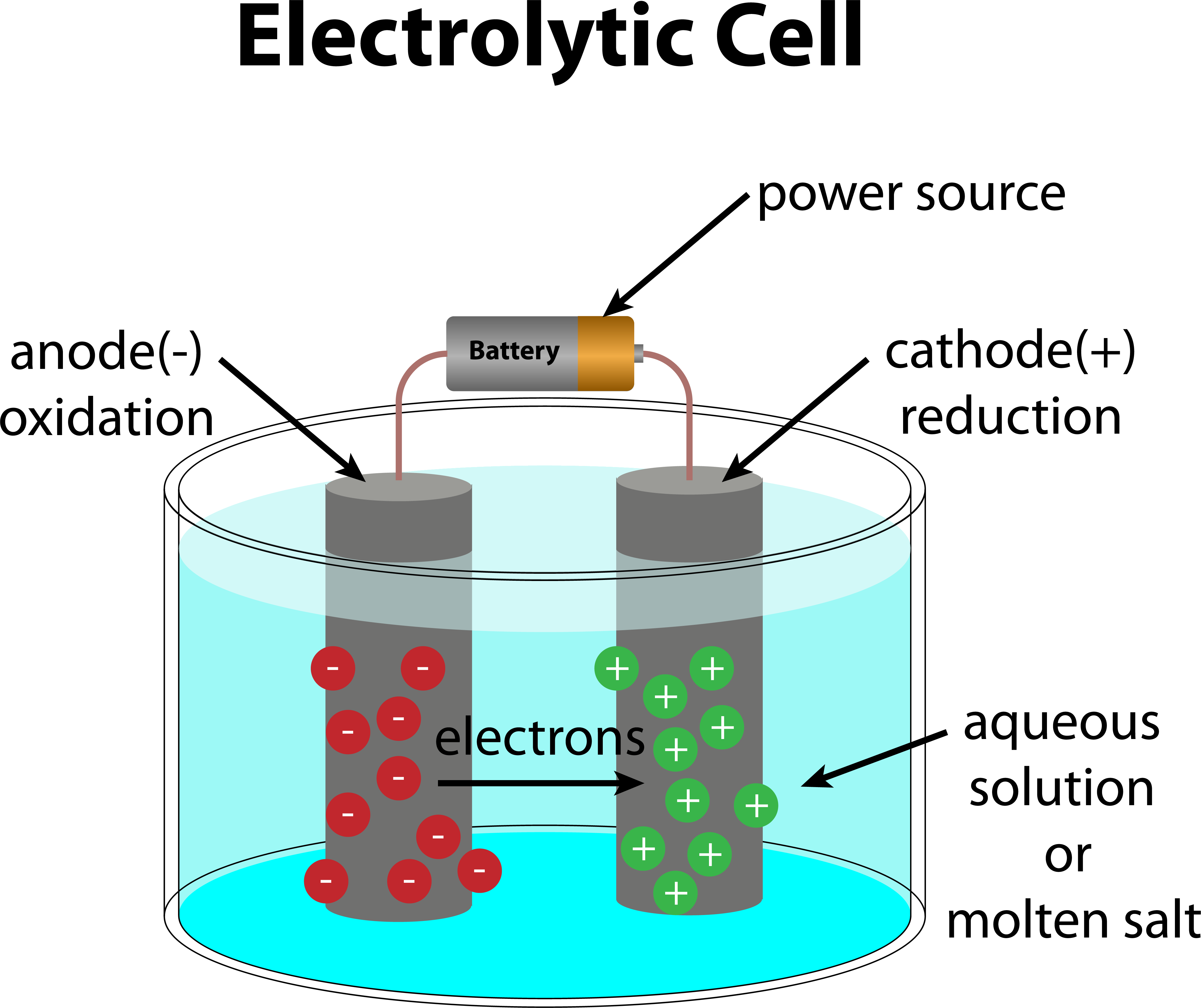
Galvanic Vs Electrolytic Cell . the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. Compare and contrast galvanic cells. learn the basics of redox reactions and how to apply them to electrochemical cells. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode.
from www.yaclass.in
learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. learn the basics of redox reactions and how to apply them to electrochemical cells. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. Compare and contrast galvanic cells.
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science
Galvanic Vs Electrolytic Cell learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. Compare and contrast galvanic cells. learn the basics of redox reactions and how to apply them to electrochemical cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells.
From
Galvanic Vs Electrolytic Cell learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. learn the basics of redox reactions. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. Web. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. learn the key. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell Compare and contrast galvanic cells. the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn the basics of redox reactions and how to apply them to electrochemical cells. Compare and contrast galvanic cells. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. electrolytic cells. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. the. Galvanic Vs Electrolytic Cell.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference Galvanic Vs Electrolytic Cell learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. Compare and contrast galvanic cells. learn the basics of redox reactions and how to apply them to electrochemical cells. electrolytic cells. Galvanic Vs Electrolytic Cell.
From patrickrgibson.blob.core.windows.net
A Galvanic Cell And The Balanced Equation For The Spontaneous Galvanic Vs Electrolytic Cell electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. learn the basics of redox reactions and how to apply them to electrochemical cells. the main distinction is. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. learn the basics of redox reactions and how to apply them to electrochemical cells. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell Compare and contrast galvanic cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. Compare and contrast galvanic cells. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn the basics of redox reactions and how to apply them to electrochemical cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. the main distinction is that galvanic cells. Galvanic Vs Electrolytic Cell.
From allthedifferences.com
What Is The Difference Between Electrolytic Cells And Galvanic Cells Galvanic Vs Electrolytic Cell learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. learn the key differences between. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. Compare and contrast galvanic cells. the main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic. electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge,. Galvanic Vs Electrolytic Cell.
From
Galvanic Vs Electrolytic Cell learn how voltaic cells, also known as galvanic cells, use the energy released by redox reactions to produce electric. Compare and contrast galvanic cells. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the. Galvanic Vs Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Galvanic Vs Electrolytic Cell Compare and contrast galvanic cells. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. learn the basics of redox reactions and how to apply them to electrochemical cells. learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells. learn how. Galvanic Vs Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Galvanic Vs Electrolytic Cell electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. learn the key differences between galvanic and electrolytic cells, such as the direction of current, the role of. Web. Galvanic Vs Electrolytic Cell.